Dipotassium ethylenediamine tetraacetate, also known as EDTA K2, has the molecular formula C10H18K2N2O10. Tripotassium ethylenediamine tetraacetic acid, also known as EDTA K3, has a molecular formula of C10H13K3N2O8.
EDTA-K2 is used as a vacuum Blood Collection Tube additive, in the preparation of toilet deodorant, in liquid analysis as a complexing agent, and the preparation of metal polish. EDTA K3 is used in complex metal ions and metal separation, in addition to anticoagulant tubes, detergents, blood anticoagulants, etc.
In many application fields, the use of anticoagulants in the blood vessels is more common. The principle of anticoagulation is to use EDTA Tube to chelate calcium ions in the blood, to achieve an anticoagulation effect.
Table of Contents
What is EDTA Tube?
This is the anticoagulant used in most hematology procedures (such as counting and identifying blood cells, blood typing, etc.)
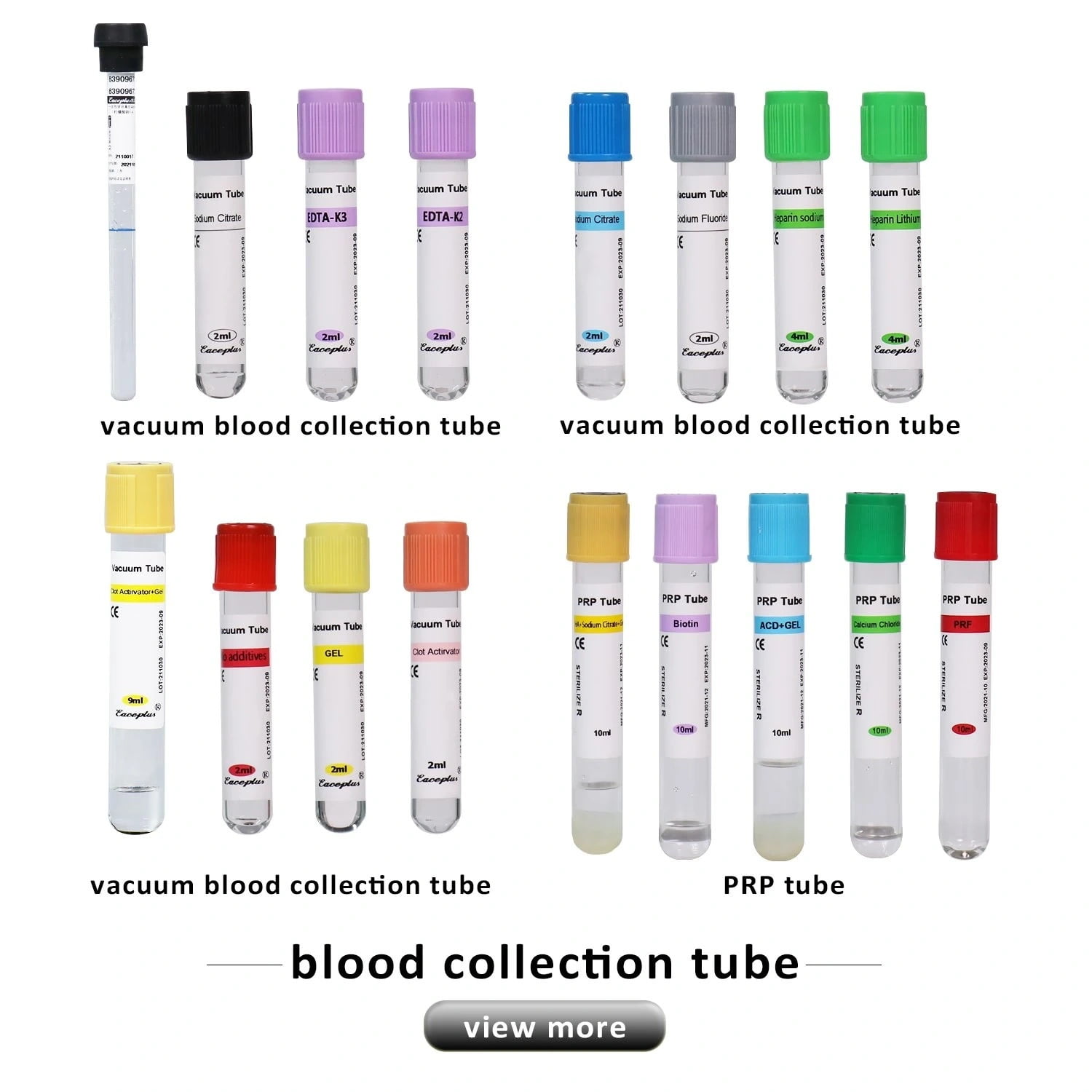
The Use of Different Vacuum EDTA Tube
| Volume/ml | Size/mm | Additive | Separator | Material | Cap Color | Closure |
| 2ml | 13X75 | EDTA K2 / EDTA K3 | / | PET/GLASS | Purple Cap Tube | Safety Cap+Rubber or Rubber Stopper |
| 3ml | 13X75 | EDTA K2 / EDTA K3 | / | |||
| 4ml | 13X75 | EDTA K2 / EDTA K3 | / | |||
| 5ml | 13X75 | EDTA K2 / EDTA K3 | / | |||
| 6ml | 13X100 | EDTA K2 / EDTA K3 | / | |||
| 7ml | 13X100 | EDTA K2 / EDTA K3 | / | |||
| 8ml | 16X100 | EDTA K2 / EDTA K3 | / | |||
| 9ml | 16X100 | EDTA K2 / EDTA K3 | / | |||
| 10ml | 16X100 | EDTA K2 / EDTA K3 | / | |||
| If You Need Customized Vacuum Blood collection tube, Please Contact Us | ||||||

What is the difference between K2 EDTA and K3 EDTA?
The anticoagulant EDTA (ethylenediaminetetraacetic acid) is commonly used to preserve blood samples. K2 EDTA and K3 EDTA are two formulations of EDTA. Although both variants prevent blood from clotting, there are notable differences between K2 EDTA and K3 EDTA. A reliable laboratory test requires a thorough understanding of these differences.
| Features | K2 EDTA | K3 EDTA |
|---|---|---|
| Anticoagulant Properties | The solution is dried on the interior surface of the tubes | An alternative anticoagulant of K2 EDTA |
| Potassium Ions | Contains 2 potassium ions | Contains 3 potassium ions |
| Chemical Composition | Dipotassium ethylenediaminetetraacetic acid | Tripotassium ethylenediaminetetraacetic acid |
| Presentation | The solution is dried on the interior surface of the tubes | The solution comes as a liquid |
Chemical composition
There is a difference in composition between K2 EDTA and K3 EDTA. In K2 EDTA, calcium ions in the blood are chelated by dipotassium salts of EDTA, which act as anticoagulants. The K3 EDTA product, on the other hand, contains tripotassium salt of EDTA and a preservative, usually potassium oxalate or potassium fluoride.
Anticoagulant Efficiency
K2 EDTA and K3 EDTA bind calcium ions to prevent blood clots. However, K3 EDTA offers enhanced anticoagulant properties compared to K2 EDTA due to its additional preservative component. Potassium oxalate or potassium fluoride in K3 EDTA inhibits glycolysis, further preserving blood samples.
Laboratory Applications
The choice between K2 EDTA and K3 EDTA is contingent upon specific laboratory requirements. K2 EDTA is extensively used in hematological testing, maintaining cell morphology, and facilitating accurate cell counting. Conversely, the superior anticoagulant and glycolysis inhibition properties of K3 EDTA make it preferable for specialized tests such as blood glucose, lactate, or pyruvate measurements.
Effects on Laboratory Tests
Despite both variants offering reliable anticoagulation, their distinct compositions may influence laboratory assays differently. The presence of potassium oxalate or potassium fluoride in K3 EDTA could interfere with specific assays, particularly those involving calcium ions or fluoride detection. Hence, laboratories must assess testing needs and adhere to guidelines when selecting the appropriate anticoagulant to ensure result accuracy.
Sample Stability
Both K2 EDTA and K3 EDTA contribute to sample stability over time. However, owing to the supplementary preservative component, K3 EDTA offers marginally better stability, particularly in inhibiting glycolysis. This attribute proves advantageous when there are delays between sample collection and subsequent analysis.
What are the features of the K2 and K3 Data Blood Collection Tubes?
- Numerical Index Design: The tubes are designed with a numerical index, ensuring easy separation during blood collection and subsequent analysis. This design feature enhances efficiency while minimizing tube shedding risk.
- Anticoagulant Fluid: These blood tubes are manufactured with anticoagulant fluid, which provides robust protection for the sample blood. This ensures the integrity of the blood sample during storage and analysis.
- Dipotassium Ethylenediaminetetraacetic Acid (EDTA): The presence of dipotassium EDTA in the tube makes it particularly suitable for a range of hematology tests, including whole blood tests, platelet counts, lymphocyte counts, and basophil tests. EDTA helps prevent blood clotting by chelating calcium ions, thereby preserving the cellular components of the blood for accurate analysis.
- Versatile Production Options: The K2 K3 EDTA blood collection tube can be produced in various configurations, including vacuum and gel-containing forms. This versatility allows healthcare professionals to choose the best tube format based on their specific testing requirements and preferences.
K2 EDTA and K3 EDTA Use
Although both can be used as vascular anticoagulants, their anticoagulant abilities are different. The key point is the ability of the main calcium complexation because this is also different in use.

We can observe that if in a 5% aqueous solution, the pH value of EDTA-K2 is 4.8 ±1, and the pH value of EDTA-K3 is 7.3 ±1, the difference lies in the stable existence of different pH values. The pH value of EDTA-K3 is neutral, which will not affect the pH value of blood.
At the same time, the ability of complex calcium ions is stronger than EDTA -K2, and the anticoagulant effect is also more muscular, but if EDTA – K3 is used as a liquid additive, it will lead to the dilution of blood samples. All blood items measured directly, such as hemoglobin, red blood cells, white blood cells, and platelet counts, are lower than those measured with EDTA -K2.
There were no clinically significant differences when comparing EDTA-K3 glass tubes with EDTA-K2 plastic tubes.
Final Summary
The two types of anticoagulants used in routine hematological tests are K2 EDTA and K3 EDTA. Blood counts are still controversial because of their effect on them. K2 EDTA contains two chelated potassium ions, while K3 EDTA contains one chelated potassium ion. The potassium ions in this compound are chelated. For effective anticoagulation, all tubes must be inverted 8–10 times, regardless of the EDTA salt used Blood and anticoagulant are thoroughly mixed.
FAQs
What is an EDTA tube used for?
EDTA tubes are primarily used in medical and laboratory settings for blood collection and preservation. The EDTA (Ethylenediaminetetraacetic Acid) in the tube acts as an anticoagulant, preventing blood from clotting and preserving the integrity of the sample for various diagnostic tests.
What is the use of K2 EDTA?
K2 EDTA, also known as dipotassium EDTA, is commonly used as an anticoagulant in blood collection tubes. Its primary function is to bind calcium ions in the blood, thereby preventing coagulation and ensuring that blood samples remain in a liquid state for laboratory analysis.
What is a K2EDTA tube used for?
A K2 EDTA tube is specifically designed for blood collection and preservation in clinical laboratories. The tube contains:
- K2 EDTA as an anticoagulant.
- Making it suitable for a wide range of hematological tests, including complete blood count (CBC).
- Blood cell morphology examinations.
- Hemoglobin analyses.
What is the Color of the EDTA Tube?
The color of EDTA tubes typically varies depending on the manufacturer, but they are commonly found in lavender or purple hues. This distinctive color helps healthcare professionals identify the tubes easily and ensures that the correct anticoagulant is used for blood collection.